Do you know Thomas Young ?
A prolific scientist and polymath, and a physician by training, he is responsible for the definition of Young’s modulus, which characterizes the elastic deformation of a material as a function of the stress applied to it. He was also the first to describe the phenomenon of accommodation, which allows the human eye to see clearly at different distances by modifying the curvature of the lens.
As a renowned Egyptologist, he made a major contribution to the interpretation of hieroglyphs, before their complete decipherment by Champollion in 1822.
Another of these major achievements, which brings us to today’s topic, was the discovery of light diffraction through slits, which allowed him, by observing interference fringes, to deduce the wave nature of light.
The diffraction used to measure the size of airborne aerosol particles is based on the use of a 633 nm helium-neon laser (see figure below). A first lens directs a parallel and coherent beam towards the atomized particles. The diffracted beam is collected on a detector using a second lens. The intensity and spacing of the interference fringes are used to determine the diffraction angle of the particles, which is itself characteristic of the size of these particles.
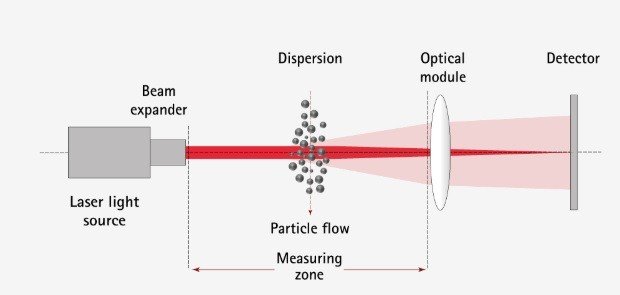
The LEREM proposes, using a reference laser granulometer for measuring the size of particles suspended in the air, a Malvern Spraytec SP2000 granulometer, to determine the particle size distribution, according to the Fraunhofer model, of aerosol generators (compressed and liquefied gases) and pump bottles, in compliance with the standard on particle size analysis ISO 13320: 2020.
The main characteristic results of the size distribution are the median diameter Dv(50), the volume mean diameter D[4][3], the percentiles Dv(10) and Dv(90), as well as the respirable fraction %V < 10 µm, necessary in the context of toxicological validations.
The applications are numerous and range from aerosol spray quality verification (cosmetics & cleaning products) to regulatory compliance assessment (pharmaceuticals & agriculture).